Cardiology
In 2015 PediaWorks, SCAI, CCISC and Children’s National Medical Center conducted a device needs survey of pediatric interventional cardiologists as a public service. The project’s goal is to provide a comprehensive assessment of clinical need and to spur the development of new devices. The following results reflect the opinions of 68 respondents from eight countries and have been presented to the FDA, NIH and several medical device firms. The raw data file is available here: 2015 Cardiology Needs Survey and may be used without permission. Please contact Tim Moran with questions or comments- tim at pediaworks org
Q1. Please rank the following in order of most needed (lowest number is highest need).
| Average |
Ranked #1 Count |
Ranked #2 Count |
Device Need |
| 2.4 | 25 | 10 | Bioresorbable stents for pulmonary artery stenosis and aortic coarctation |
| 3.7 | 14 | 15 | Covered stents in very large sizes |
| 5.1 | 3 | 12 | Transcatheter pulmonary / tricuspid valves in larger sizes |
| 5.1 | 7 | 4 | Tubes and patches that can be dilated |
| 5.5 | 4 | 4 | PDA devices for very tiny premature infants |
| 6.5 | 2 | 4 | Perimembranous VSD occluder devices |
| 6.7 | 4 | 4 | One piece front loading self-sheathed stent delivery systems |
| 6.9 | 1 | 4 | Catheter delivered pulmonary flow limiters to replace surgical PA banding |
| 7.1 | 0 | 1 | Stents and delivery systems for PDA stenting in infants |
| 7.4 | 0 | 1 | Wires with variable stiffness characteristics that can be altered in vivo |
| 9.2 | 1 | 1 | 3Fr. long sheaths |
| 10.3 | 0 | 0 | Flexible microtome cutting balloon |
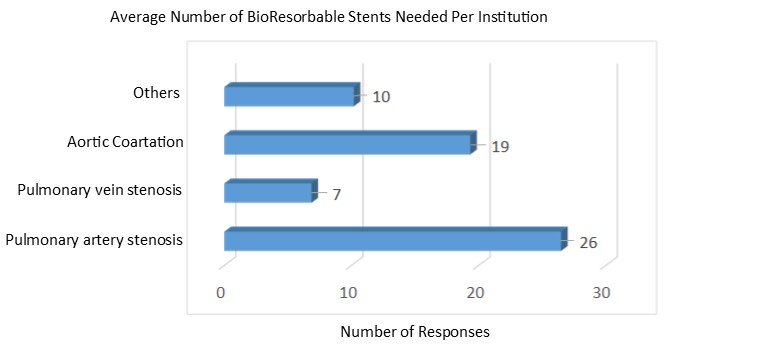
Q2. If a bioresorbable stent were safe and efficacious, please list the number your institution would likely use per year to treat the following conditions:
Q3. Please list the two most common bioresorbable stent diameters required to treat PAS and CA:
Q4. For PDA stents please define maximum expanded diameter needed:
Q5. For PDA stents please define the maximum expanded length:
Q6. For PDA stents please define desired material:
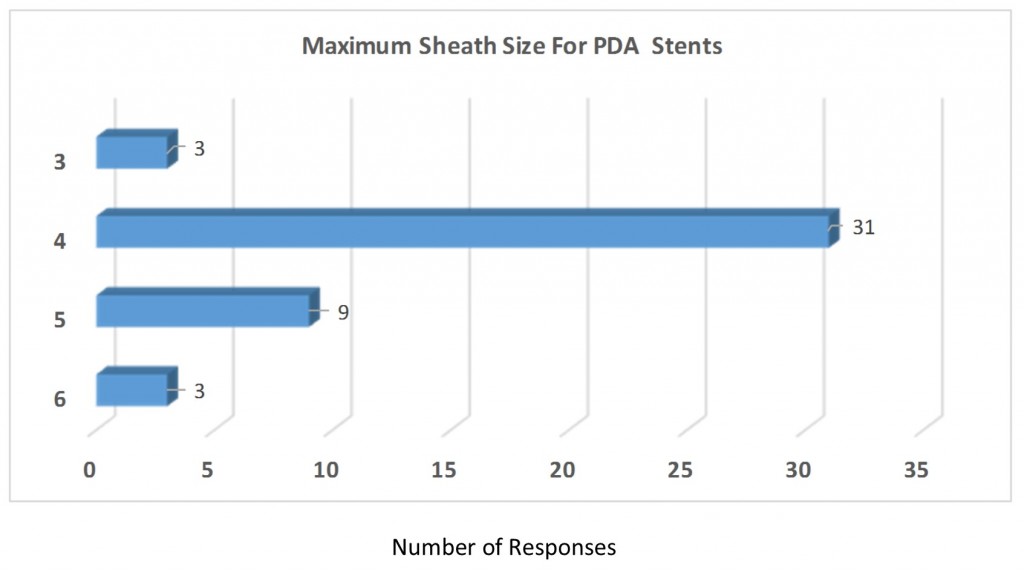
Q7. Please define the maximum sheath size for PDA stents:
Q8. Please define the maximum diameter for covered stents:
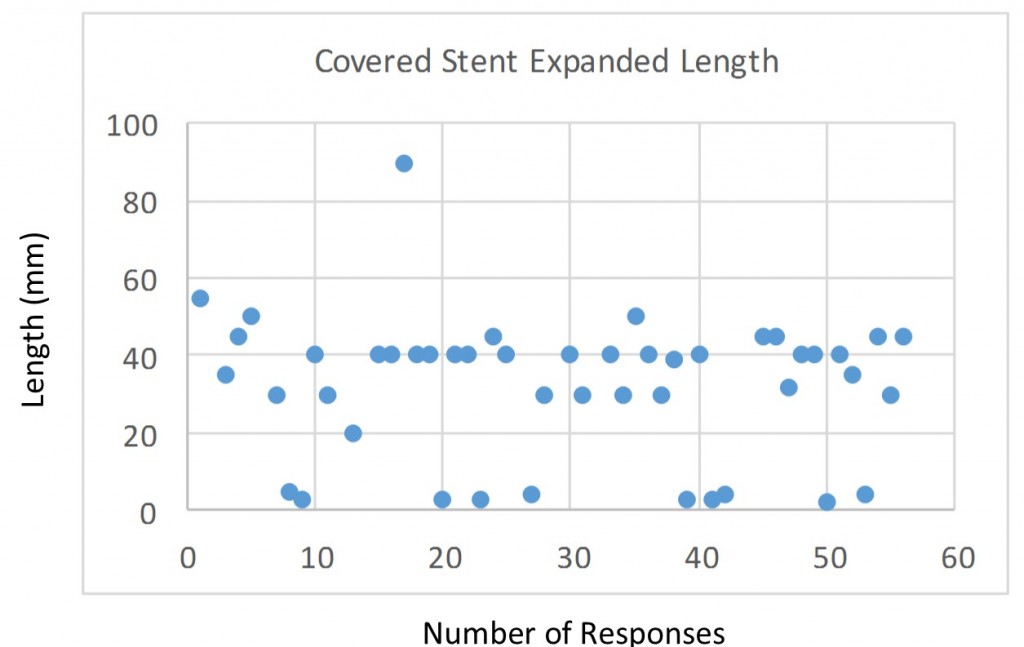
Q9. Please define maximum expanded length for covered stents:
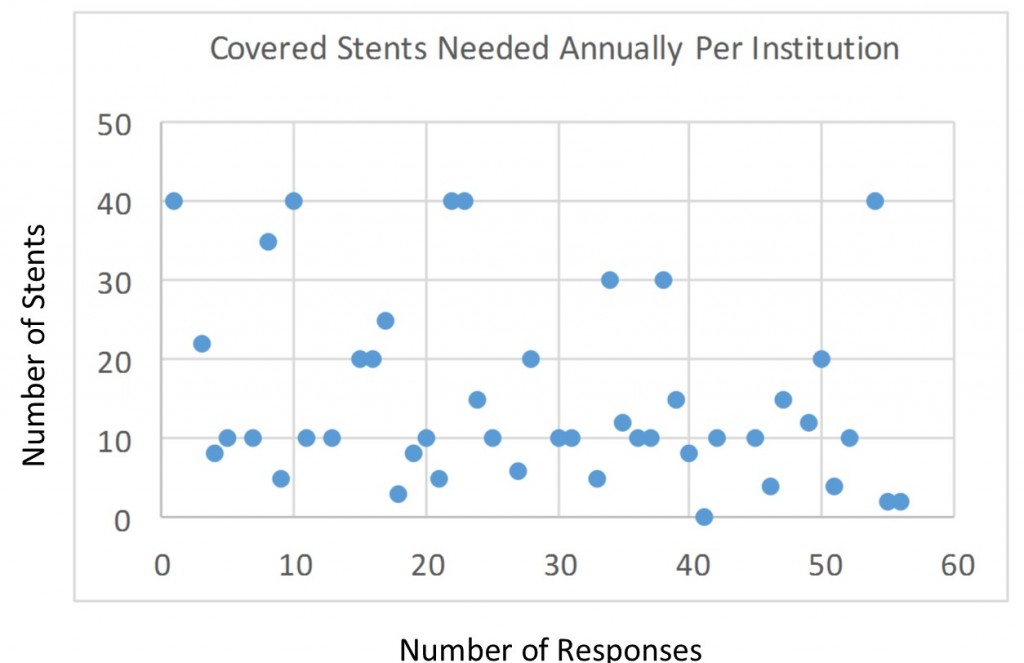
Q10. Please define the number of covered stents needed annually at your institution:
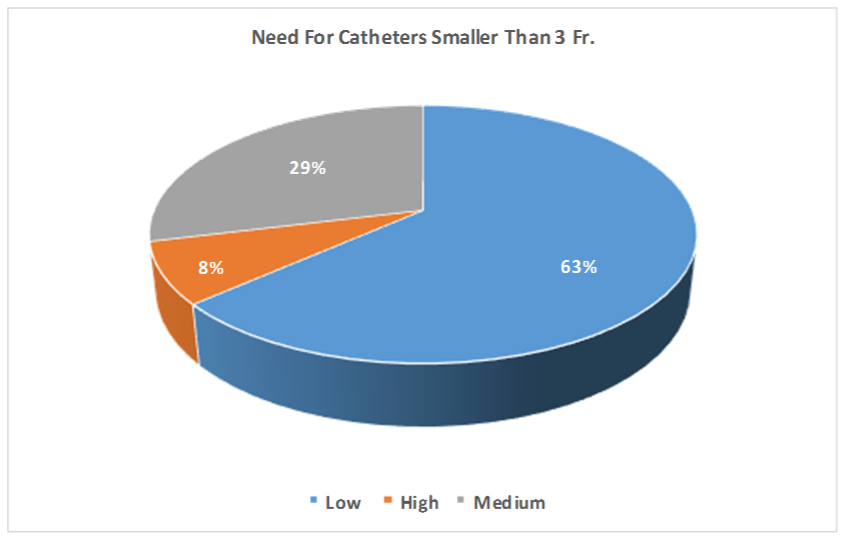
Q11. Please rate the need for catheters smaller than 3 Fr.:
Q12. Please list a new or modified device that would affect the greatest number of your patients:
| – Bioresorble stent (X12) | |||||||||
| – PDA device for premies (X7) | |||||||||
| – Larger pulmonary and tricuspid valves (x5) | |||||||||
| – Covered stents (X5) | |||||||||
| – Perimembranous VSD devices (x3) | |||||||||
| – Bioresorbable septal occluders (x3) | |||||||||
|
Q13. Please list a new or modified device that would have the greatest impact on your patients’ morbidity:
| – Bioresorbable stents (x11) | |||||||||||||||
| – Covered stents (X11) | |||||||||||||||
| – PDA closure and delivery device for premature infants (X2) | |||||||||||||||
| – Larger percutaneous pulmonary valve (2X) | |||||||||||||||
|
Q14. Please list desired simple modifications to current devices:
| – Premounted stents (X6) |
| – Covered stents (X4) |
| – Bioabsorbable stent (X2) |
| – A better balloon expandable stent; remounted, doesn’t shorten excessively, can be further dilated and goes through a reasonable size sheath |
| – Amplatzer Duct Occluder 7/5 doesn’t exist. |
| – Another self centering ASO device |
| – Bigger transcatheter valves |
| – Carrot attached to long sheaths simplifying stent delivery |
| – Covered stent even material that can allow us to make a covered stent in the lab on a case by case basis |
| – Delivery sheath size, flexibility and traceability; Guide wire in vivo modification of performance features |
| – Ductal occluders shaped like an ADO I whixch can be deployed from the arterial end. |
| – Finer softer nitinol mesh on Amplatzer septal occluder |
| – Hemostatic valve that accomodates large catheters/stents. To add onto an existing sheath, either by copping the existing one off and using the new valve or by introducing the new one through the existing valve. The current ones are very tight and can strip off a mounted stent. Also a 5 and 6 Fr. transseptal sheath with a bright tip marker at the end. |
| – Large diameter preloaded stents |
| – Larger percutaneous pulmonary valve |
| – Larger pulmonary valves for replacement therapy in all forms of RVOT dysfunction |
| – Make stents retrievable |
| – More minaturization of current ADO I device to use in premature infants. If constructed out of material similar to AVP IV or ADO II but in the shape of AVP II, I think premie PDA’s could be closed via an 0.038 lumen catheter. |
| – Nucleus shape balloons in small sizes for ductal stenting |
| – “Open ring”, “growth” or resorbable small stents |
| – PDA bioresorbable device |
| – PDA device |
| – Pre-mounted Palamz Genesis and Palmaz XL stents on large balloons (eg 18, 20, 22, 24 mm) |
| – VSD device |
| – Wider variety of stent lengths so that we don’t have to ovelap side branches in PA stenting |
| – With the success of the 3.3 Fr sheath and catheters, work should be performed on a 2.2 Fr sheath and catheter |
Q15. Please list devices available in other countries that you would like available in yours:
| – Covered stents (X21) |
| – Large diameter covered stent (X6) |
| – Melody pulmonary valve (X2) |
| – All of them! |
| – ASD occluders with improved delivery system that allows retrievability following implantation and eliminatiion of tension via the delivery cable |
| – AVP III (vascular plug 3-oval plug), LifeTech devices, Occlutech devices |
| – Better stronger, covered stents; Better deployable occlusion plugs |
| – Biodegradable stent |
| – Larger pulmonary valves |
| – Other septal occluders, percutaneous valves |
| – Perimemb VSD device (PFM) |
| – Perimembranous VSD device |
| – RVOT infundibular reducer for pulmonary valve implantation |
| – Stents approved for pediatric congenital use and covered stents in the USA |
| – Transcatheter pulmonary valve, self-expanding, name escapes me; or ADO II-AS |
| – Vascular plug III |
Q16. Please describe any other product needs:
| – Better balloon-tipped wedge and angiographic catheters. Only 2 suppliers in the US. The Arrow catheters have bad balloons. The other is not very steerable and has inconsistent bore sizes. |
| – Double lumen central lines with high flow in 3 Fr size, 5 Fr high flow renal replacement catheters |
| – Percutaneous valves for other than pulmonary application |
| – Premounted stents of adequate size to be dilated to adult size |
| – Small PDA occluders for premature infants, Improved perimembranous VSD occluders |